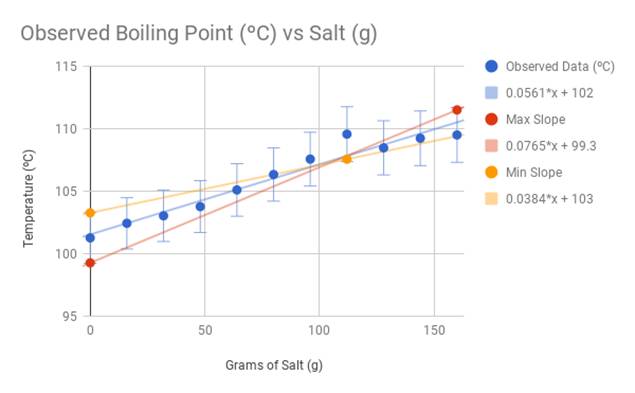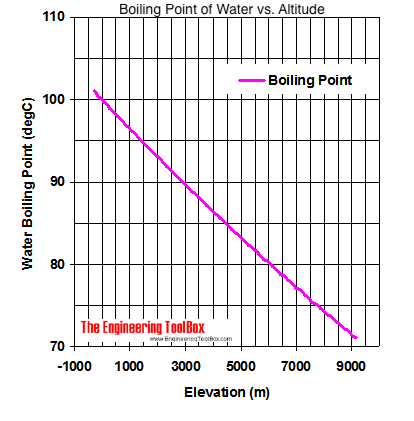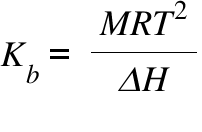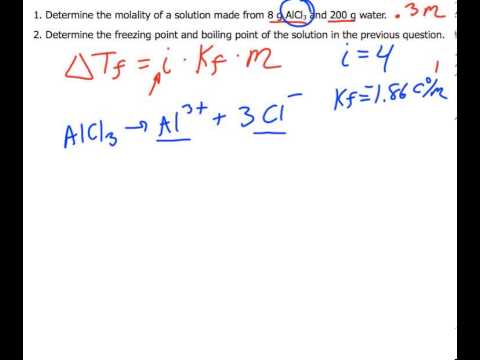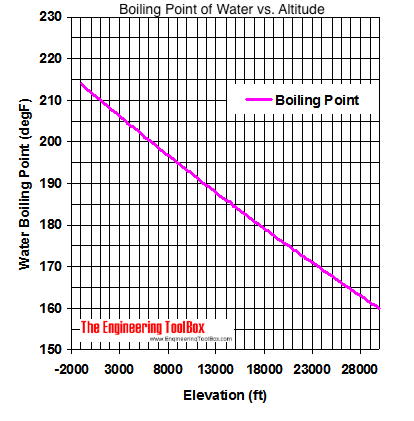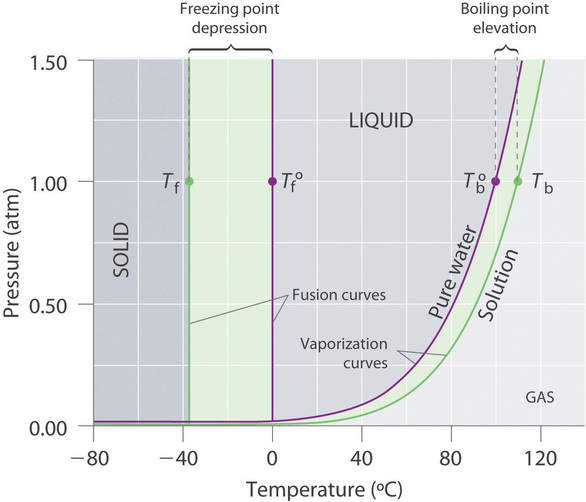
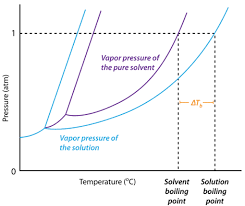
13.8: Freezing-Point Depression and Boiling-Point Elevation of Nonelectrolyte Solutions - Chemistry LibreTexts

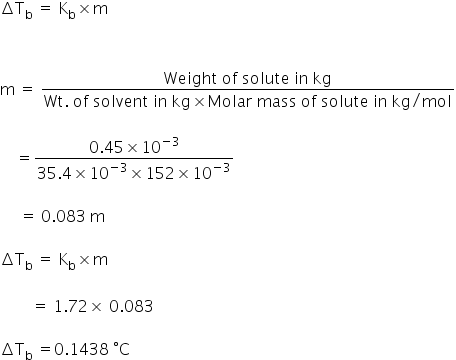
SOLVED: The elevation in boiling point, when 0.30 g of acetic acid is dissolved in 100 g of benzene is 0.0633OC. Calculate the molecular weight of acetic acid from this data. What

Calculations Involving Colligative Properties Freezing Point Depression and Boiling Point Elevation Calculations. - ppt download
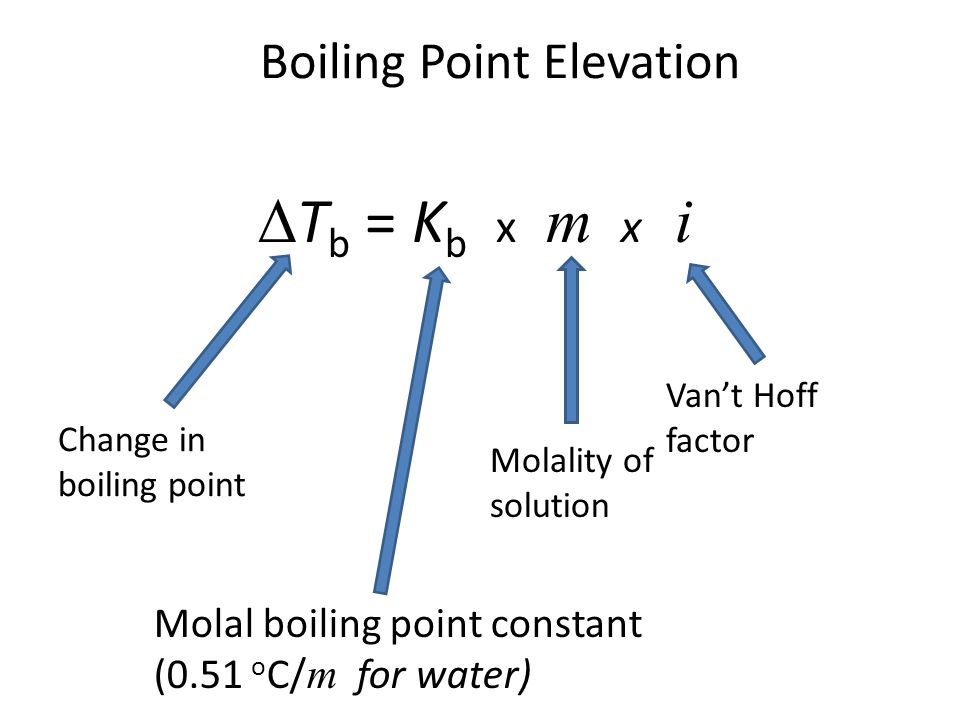
How to Calculate and Solve for Van't Hoff Factor, Ebullioscopic Constant, Molality and Boiling Point Elevation | The Calculator Encyclopedia - Nickzom Blog

Calculations Involving Colligative Properties Freezing Point Depression and Boiling Point Elevation Calculations. - ppt download

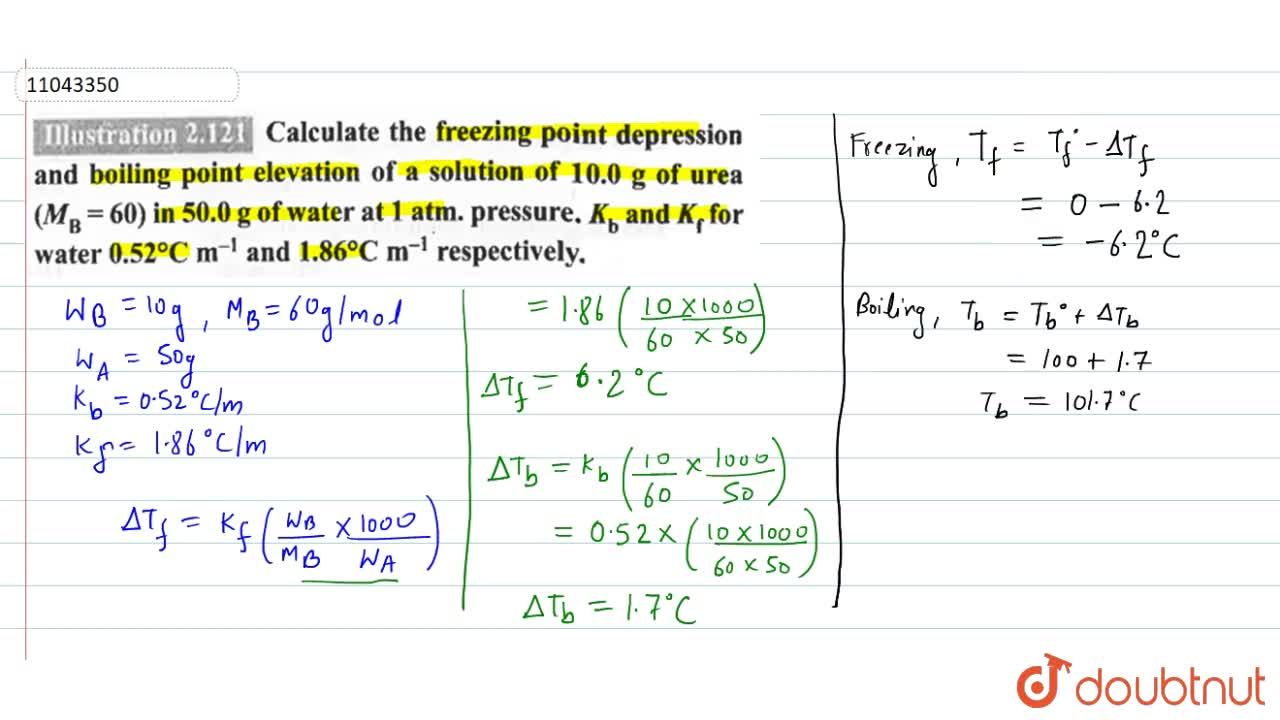
Calculate the molal elevation constant, kb for water and the boiling point of 0.1 molal urea solution. Latent heat of vaporisation of water is 9.72 kcal mol ^-1 at 373.15 K.